Ozone
State-of-art research laboratory in our main facility is actively into various application of ozone technology, product innovation, design development and extensive testing. We have a vast expericence in Plasma Science, High Voltage Engineering, Thermal Transfer Efficiencies, Mechanical CAD, PLC and Automation. Our High Voltage, High Frequency Corona Discharge enables us to achieve High Ozone Concentration of 8 - 12% by weight. Higher the concentration, better the dissolved ozone in water and lower the oxygen feed gas requirement. Built on rich sensitivites and creativity, continuous improvement and innovation is our strength and we will continue to offer products and services that bring satisfaction and evoke inspiration that exceeds the expectations of our customers.
Ozone is a Natural gas
Ozone is an unstable gas and it is made of just one thing oxygen, it has very short life, which means it reacts and disappears rapidly. Any pathogen or contaminant that can be disinfected, altered or removed via an oxidation process will be affected by ozone. It is the strongest of all molecules available for disinfection in water treatment & is second only to elemental fluorine in oxidizing power. Ozone gas produced by ozone generator, which oxidants and disinfectant for air and water treatment. At Faraday Crystal, we are focusing our efforts on various applications and markets in which we can make significant impacts.
Why Ozone?
Ozone oxidation is the most excellent and environment friendly to disinfects the water and air. It works effectively against bacteria, viruses compared to chlorine. In addition, oxidizing properties can also reduce the concentration of iron, manganese, sulfur and reduce or eliminate taste and odour problems. Ozone oxides the iron, manganese, and sulfur in the water to form insoluble metal oxides or elemental sulfur. These insoluble particles are then removed by post-filtration. Organic particles and chemicals will be eliminated through either coagulation or chemical oxidation. Ozone is unstable and it will degrade over a time frame ranging from a few seconds to 30 minutes. The rate of degradation is a function of water chemistry, pH and water temperature.

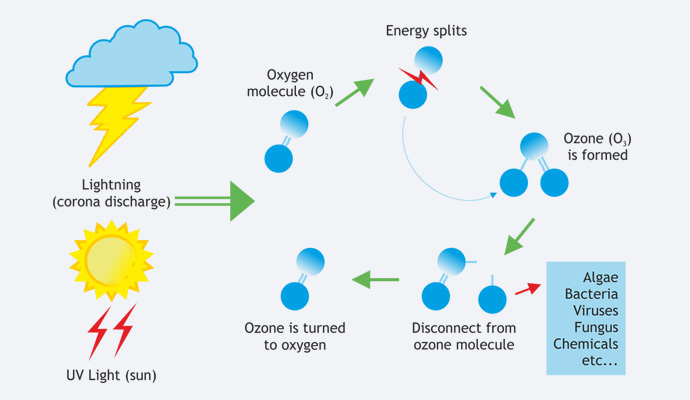
Oxygen - Ozone Cycle
Ozone is fundamentally made up by oxygen. When an oxygen molecule (O2) is exposed to electric high voltage (or UV-light in the stratosphere), the oxygen molecule (O2) is split into two oxygen atoms (O1). The resulting oxygen atom (O1) to connects with oxygen molecules (O2) and ozone (O3) is formed. Ozone then reacts with other substances and the single oxygen atom (O1) disconnects from the ozone molecule (O3), which then again turns into an oxygen molecule. The ozone is injected into the water or air stream, where it inactivates contaminants by actually rupturing the organism call wall. At the heart of a corona discharge ozone system is the dielectric. The electrical charge is diffused over this dielectric surface, creating an electrical surface, creating an electrical field or "Corona".
Ozone Benefits
Ozone can be used in many applications and it is generated on site from oxygen, which eliminates the need to haul chemicals or other dangerous products. In many applications ozone saves money, environment & time. Ozone is much healthier & safer to use than harsh caustic chemicals & it does an excellent job. There are many applications where thermo oxidation is used to disinfect or clean. Ozone can do the same job as thermo oxidation, but with the advantage of working in cold environments, this provides savings in energy & money.
- Ozone is 51% more powerful on bacterial cell walls than chlorine
- Ozone kills bacteria 3100 times faster than chlorine
- Ozone is the most powerful broad spectrum microbiological control agent available
- Ozone eliminates the use of hot water and conventional sanitizer
- Ozone virtually eliminates all chemical usage
- Ozone is chemical-free; it produces NO toxic by-products
- Ozone has full FDA-approval for direct-food contact application
- Ozone is clean and environment-friendly, its only by-product is oxygen
- Ozone is extremely effective as a disinfectant at relatively low concentrations
- Ozone is generated on site eliminating the transporting, storing and handling of hazardous materials
- Ozone is very inexpensive to produce and has an unlimited supply
- Ozone is much safer for employees than any conventional chemicals
- Ozone extends the shelf life of food products
- Ozone permits recycling of wastewater
- Ozone reduces Biological Oxygen Demand (BOD)
Organisms killed by Ozone
One benefit is the variety of microbes ozone can kill with a small dose and residual. Many factors determine the residual, but generally, the higher the ozone production, the higher the residual and the longer it will last in the water. Required residual is dictated by the amount and type of microbes to be killed. When ozone degrades, it reverts back to oxygen, thus it is safe and not a chemical hazard to people, equipment or the environment.
Bacteria
- Achromobacter butyri NCI-9404
- Aeromonas harveyi NC-2
- Aeromonas salmonicida NC-1102
- Bacillus anthracis
- Bacillus cereus
- B. coagulans
- Bacillus globigii
- Bacillus licheniformis
- Bacillus megatherium sp.
- Bacillus paratyphosus
- B. prodigiosus
- Bacillus subtilis
- B. stearothermophilus
- Clostridium botulinum
- C. sporogenes
- Clostridium tetoni
- Cryptosporidium
- Coliphage
- Corynebacterium diphthriae
- Eberthella typhosa
- Endamoeba histolica
- Escherichia coli
- Escherichia coli
- Flavorbacterium SP A-3
- Leptospira canicola
- Listeria
- Micrococcus candidus
- Micrococcus caseolyticus KM-15
- Micrococcus spharaeroides
- Mycobacterium leprae
- Mycobacterium tuberculosis
- Neisseria catarrhalis
- Phytomonas tumefaciens
- Proteus vulgaris
- Pseudomonas aeruginosa
- Pseudomonas
- fluorscens (bioflims)
- Pseudomonas putida
- Salmonella choleraesuis
- Salmonella enteritidis
- Salmonella typhimurium
- SalmonSalmonella typhimurium
- Salmonella typhosa
- Salmonella paratyphiSarcina lutea
- Seratia marcescens
- Shigella dysenteriae
- Shigella flexnaria
- Shigella paradysenteriae
- Spirllum rubrum
- Staphylococcus albus
- Staphylococcus aureus
- Streptococcus 'C’
- Streptococcus faecalis
- Streptococcus hemolyticus
- Streptococcus lactis
- Streptococcus salivarius
- Streptococcus viridans
- Torula rubra
- Vibrio alginolyticus & angwillarum
- Vibrio clolarae
- Vibrio comma
- Virrio ichthyodermis NC-407
- V. parahaemolyticus ella typhosa
- Salmonella paratyphiSarcina lutea
- Seratia marcescens
- Shigella dysenteriae
- Shigella flexnaria
- Shigella paradysenteriae
- Spirllum rubrum
- Staphylococcus albus
- Staphylococcus aureus
- Streptococcus 'C’
- Streptococcus faecalis
- Streptococcus hemolyticus
- Streptococcus lactis
- Streptococcus salivarius
- Streptococcus viridans
- Torula rubra
- Vibrio alginolyticus & angwillarum
- Vibrio clolarae
- Vibrio comma
- Virrio ichthyodermis NC-407
- V. parahaemolyticus
Fungus & Molds Spores
- Aspergillus candidus
- Aspergillus flavus (yellowish-green)
- Aspergillus glaucus (bluish-green)
- Aspergillus niger (black)
- Aspergillus terreus, saitoi & oryzac
- Botrytis allii
- Colletotrichum lagenarium
- Fusarium oxysporum
- Grotrichum
- Mucor recomosus A & B (white-gray)
- Mucor piriformis
- Oospora lactis (white)
- Penicillium cyclopium
- P. chrysogenum & citrinum
- Penicillium digitatum (olive)
- Penicillium glaucum
- Penicillium expansum (olive)
- Penicillium egyptiacum
- Penicillium roqueforti (green)
- Rhizopus nigricans (black)
- Rhizopus stolonifer
Virus
- Adenovirus (type 7a)
- Bacteriophage (E.coli)
- Coxackie A9, B3, & B5
- Cryptosporidium
- Echovirus 1, 5, 12, & 29
- Encephalomyocarditis
- Hepatitis A
- HIV
- GD V11 Virus
- Onfectious hepatitis
- Influenza
- Legionella pneumophila
- Polio virus (Poliomyelitus) 1, 2 & 3
- Rotavirus
- Tobacco mosaic
- Vesicular Stomatitis
Fungal Pathongens
- Alternaria solani
- Botrytis cinerea
- Fusarium oxysporum
- Monilinia fruiticola
- Monilinia laxa
- Pythium ultimum
- Phytophthora erythroseptica
- Phytophthora parasitica
- Rhizoctonia solani
- Rhizopus stolonifera
- Sclerotium rolfsii
- Sclerotinia sclerotiorum
Yeast
- Baker's yeast
- Candida albicans-all forms
- Common yeast cake
- saccharomyces cerevisiae
- saccharomyces ellipsoideus
- saccharomyces sp.
Protozoa
- Paramecium
- Nematode eggs
- Chlorella vulgaris (Algae)
- All Pathogenic & Non-pathogenic
Algae
- Chlorella vulgaris
- Thamnidium
- Trichoderma viride
- Verticillium albo-atrum
- Verticillium dahliae
Cysts
- Cryptosporidium parvum
- Giardia lamblia
- Giardia muris
